Prescription Drug Price Changes Through January 2024
FAYETTEVILLE, NEW YORK, UNITED STATES, February 1, 2024 /EINPresswire.com/ -- Prescription Drug Price Increases Range from 0.76% to 25.00%
The AnalySource Team has been busy tracking price changes that have taken effect since our last press release on January 8, 2024. Since then, manufacturers have reported Wholesale Acquisition Cost (WAC)* list price increases for 118 single source products at an average of 4.85%.
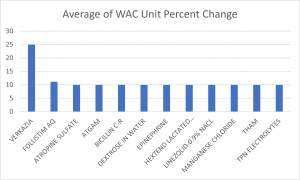
The largest WAC unit increase since January 8 was applied to the product VERKAZIA. This is a product manufactured by Eyevance/Harrow for the treatment of acute vernal keratoconjunctivitis. This is the first list price increase that this product has taken since 2022. Meanwhile, the smallest increase was attributed to Novartis’ LEQVIO, with a 1.25% increase. LEQVIO is a Small Interfering RNA Agent, that last took a WAC unit price increase of 1.25% in July 2023. Thus far in 2024, Incyte Corporation’s JAKAFI still has the lowest WAC unit price increase for the new year, with their 0.76% list price increase posted prior to our latest press release.
In the beginning of the month, we looked at price increases for products in the IQVIA Top 200 Prescription Products list for 2022. Since our last press release 14 products have taken WAC unit increases. This features well-known products such as COSENTYX by Novartis and XARELTO by Janssen Pharmaceuticals.
Overall, there have been 719 single source products that have enacted WAC unit price increases. These increases were issued by 203 labelers at an average increase of 4.92%.
As reported in our first press release, Genentech had enacted price increases on 23 different single source products. Novartis has since changed the WAC unit price of 26 unique single source products for an average price increase of 5.69%.
Please note that these price changes affect list prices, or Wholesale Acquisition Cost** (WAC), that are set by the drug manufacturers without considering rebates, insurance, and other discounts that may be available.
Source
AnalySource® as of January 31, 2024 - Reprinted with permission by First Databank, Inc. All rights reserved. © 2024
* Single source products are determined by the Center for Medicare and Medicaid Services, with increases in WAC Unit Pricing with effective dates between 1/1/2024 – 1/31/2024.
** First Databank, Inc Drug Pricing Policy: https://www.fdbhealth.com/drug-pricing-policy
About DMD America, Inc
AnalySource® is a registered trademark and drug pricing data solution service of DMD America, Inc. Since 1996, data has been made available in cooperation with First Databank, Inc., a subsidiary of the Hearst Corporation. Our service is licensed by subscription, with global clients including biotech, pharmaceuticals, government agencies, consultancies, academia, and more.
About First Databank (FDB)
First Databank (FDB) is the leading provider of drug and medical device knowledge that helps healthcare professionals make precise decisions. We empower our information system developer partners to deliver valuable, useful, and differentiated solutions used by millions of clinicians, business associates, and patients every day. For more than four decades, our medical knowledge has helped improve patient safety, operational efficiency, and healthcare outcomes. For a complete look at our solutions and services, please visit www.fdbhealth.com.
Daniel Miccio
DMD America, Inc.
+1 315-400-2289
email us here
Visit us on social media:
Twitter
LinkedIn
Distribution channels: Business & Economy, Healthcare & Pharmaceuticals Industry, Insurance Industry, Politics, U.S. Politics
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release