LifeBioBRAIN Found to be a Usable Cognitive Screening Prototype

LifeBioBRAIN cognitive screening prototype
LifeBio and Brown University collaborate to engage patients living with mild cognitive impairment or early-stage dementia to test usability of new app
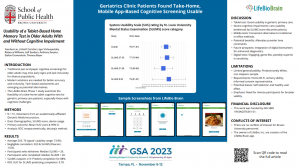
MARYSVILLE, OH, UNITED STATES, February 15, 2024 /EINPresswire.com/ -- A study conducted by Brown University researchers established that geriatric primary care clinic patients, with mild cognitive impairment or dementia, found the LifeBioBRAIN™ cognitive screening prototype usable.
LifeBio will further develop LifeBioBRAIN™ for use by primary care providers (PCPs) and outpatient physicians (neurologists, psychiatrists, neuropsychologists) to utilize as a cognitive screening tool for at-home or in-clinic screening to determine the need for further comprehensive neurological and neuropsychological assessments.
As LifeBioBRAIN (LBB) progresses in development, the goal is for it to be used similarly to traditional pen and paper screening tools (still widely in use today) which could then prompt referral for further neuropsychological testing and/or consultation with a neurologist or neuropsychologist. Development will focus on improving the game-like interface of LBB to identify cognitive function (in areas such as memory, attention, executive function, etc.) while also building out biomarker capture capabilities.
The U.S. population aged 65 or over is expected to be 75 million by 2030; this size of this demographic will drive enormous growth in the demand for health care services for older people, many of whom will at some point develop Alzheimer’s disease or related dementias.
In the United States, the number of individuals living with Alzheimer’s Disease and Related Dementias (AD/ADRD) is expected to increase from 6.7 million to 7.2 million by 2025 and reach 11.2 million by 2040. Currently, $345 billion is spent annually on the costs of care associated with AD in the U.S. ($222 billion by Medicare, Medicaid alone). In 2021, the annual global cost of dementia was estimated to be more than $1.3 trillion and is expected to rise to $2.8 trillion by 2030. This figure includes unpaid care provided by family/friends, direct cost community care/residential/senior living/home care, and the direct costs of medical care (the costs of treating dementia and other conditions in primary and secondary care).
Cognitive screening for dementia will be important because patients with neurodegenerative diseases including Alzheimer’s Disease are negatively impacted by cognitive, motor, and neuropsychiatric difficulties. It is important to differentiate between normal cognition and provided adequate treatment to use possible pharma and non-pharma approaches with the patient for optimal care, thereby assisting the family caregiver or professional caregiver too.
LifeBioBRAIN holds potential for adoption across a spectrum of healthcare settings, from individual practices to large group practices, hospital networks, academic medical centers, and research projects. Early adopters are likely to include individual primary care physician offices seeking to enhance practice efficiency through LifeBioBRAIN's rapid examination capabilities.
Screening for cognitive change sooner may lead to eligibility for new drugs, participation in clinical trials, deployment of meaningful interventions, and overall better health care outcomes. Early detection of major neurocognitive disorders enables more timely deployment of pharmacologic and non-pharmacologic interventions to help both persons living with dementia (PLWD) and their professional or family caregivers. Cognitive screening tools must also become more inclusive for demographically diverse individuals and LifeBioBRAIN plans to address this need as development continues. A body of prior work has documented limitations of screenings that are not sensitive to varying socioeconomic, cultural, racial, or other differences.
Brown University researchers found that take-home mobile device-based cognitive testing is a usable strategy in older adult primary care patients across a range of cognitive function, but less viable in persons with severe cognitive impairment. Take-home mobile device-based screening could be part of a flexible cognitive testing and follow-up strategy that also includes mobile device-based testing in healthcare settings and pen-and-paper cognitive testing, depending on patient preferences and abilities.
Future development of the LBB prototype is necessary before it can be used in market. This work was funded by the National Institute on Aging SBIR program, grant number 1R43AG076341-01.
Beth Sanders
LifeBio
+1 937-303-4574
bsanders@lifebio.com
Visit us on social media:
Facebook
Twitter
LinkedIn
Instagram
Distribution channels: Book Publishing Industry, Healthcare & Pharmaceuticals Industry, Insurance Industry, Science, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release